This report presents the experimental and numerical work carried out by ECN and Hanze University of Applied Sciences on methane sorption on activated carbon, as part of their activities within the EDGaR Energy Storage project. Eleven different activated carbon types were tested. It was found that MaxSorb MSC-30 offered the highest methane mass storage density (m/m ratio). However, due to the low density of the MaxSorb MSC-30 activated carbon, the highest volumetric methane storage density (V/V ratio) was found for Brightblack. An increase of the packing density and heat conductivity significantly improves the V/V ratio and shortens the time needed to reach thermal equilibrium. In the case of the Brightblack activated carbon, a total V/V ratio of 112 was found at 12 oC and 40 bar, implying an effective storage density that is 3 times higher than for compressed methane. During the adsorption of methane on activated carbon, sorption heat is released and the temperature of the bed is increased, which negatively affects the effective V/V ratio. Temperature rises up to 70 oC were experimentally observed at higher methane inflow rates. For MaxSorb MSC-30 a temperature rise of 25 oC reduced the effective V/V ratio by about 20 %. The temperature rise of the Brightblack bed caused relatively smaller reductions in the volumetric storage density. Calculations with the validated numerical models indicated an even higher temperature increase for the full scale methane storage, reaching bed temperatures up to 137-150 oC in the case of the MaxSorb MSC-30 activated carbon. At this temperature range, the models indicate a V/V ratio fall down to 46. This performance is similar to the one offered by direct methane compression to 40 bar, and is much lower than the V/V ratio of ~ 100 that was found both experimentally and by calculations for the lab scale reactor performance. The calculations showed, that the low bed permeability can limit the gas flow during adsorption and desorption. A high reactor diameter can countervail the effect of permeability, but the higher dimensions impede the heat dissipation and thus decrease the storage efficiency. Efficient temperature control and management are very important to effectively make use of the methane storage capacity through adsorption.
DOCUMENT
Methane storage in adsorbed form is a promising way to effectively and safely store fuel for vehicular transportation or for any other potential application. In a solid adsorbent, nanometer wide pores can trap methane by van der Waals forces as high density fluid at low pressure and room temperature. This provides the suitable technology to replace bulky and expensive cylindrical compressed natural gas tanks. Activated carbons with large surface area and high porosity are particularly suitable for methane storage applications at moderate pressures. We study and test the main thermodynamic and kinetic characteristics of methane adsorption and desorption on activated carbon.
DOCUMENT
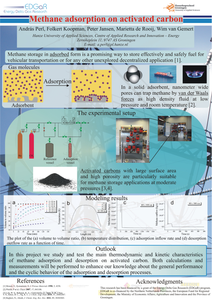
Locally produced methane, - either as biomethane or power-to-gas product, has to be stored to provide a reliable gas source for the fluctuating demand of any local gas distribution network. Additionally, methane is a prominent transportation fuel but its suitability for vehicular application depends on the ability to store an adequate amount in the onboard fuel tank. Adsorption in porous materials could enable a simple, safe and cost-effective method for storing methane at ambient temperature and at reasonably low pressure. In this project we study and test the main thermodynamic and kinetic characteristics of methane adsorption and desorption on activated carbon. Both calculations and measurements are performed to enhance our knowledge about the general performance and the cyclic behavior of the adsorption and desorption processes.
DOCUMENT

Adsorbed natural gas (ANG) storage using metal-organic frameworks (MOFs) is a promising alter- native for efficient natural gas storage at moderate pressures. However, the presence of higher alkanes in natural gas mixtures can significantly affect storage performance by reducing methane adsorption capacity. Basolite C300, a well-studied MOF, offers high volumetric methane storage, but its long-term efficiency in real-world conditions remains a challenge due to potential pore blockage from hydrocarbon accumulation. This study investigates the long-term impact of Cn≥2 alkanes on the adsorption capacity of Basolite C300. Volumetric storage capacities of methane, individual alkanes, and a natural gas mixture were measured at 20 °C. The material underwent 100 adsorption-desorption cycles to assess the progressive impact of Cn≥2 alkanes on methane storage. The experimental results revealed a 63% reduction in methane storage capacity after 100 cycles, highlighting the detrimental effect of alkane accumulation. Higher alkanes were preferentially adsorbed within Basolite C300 micropores, leading to progressive pore blockage and decreased methane uptake. These findings underscore the critical role of gas composition in ANG systems and emphasize the need for mitigation strategies, such as selective pre-adsorption or regeneration techniques, to maintain long-term storage efficiency in MOF-based gas storage applications.
DOCUMENT
Microencapsulation of cells is a promising approach to prevent rejection in the absence of immunosuppression. Clinical application, however, is hampered by insufficient insight in factors influencing biocompatibility of the capsules in humans. In the present study we exposed alginate-based capsules prepared of different types of alginate to human peritoneal fluid. Subsequently we studied the physicochemical changes of the capsule's surface by applying micro-Fourier Transform Infrared Spectroscopy. We did test alginate-beads and alginate-poly-L-lysine capsules prepared of different types of alginate. In all tested capsule formulations we found adsorption of components from human peritoneal fluid and clear physicochemical changes of the surface. These changes were alginate-dependent. The adsorption had no significant effects on the permselective properties of the capsule but we found a strong increase of TNFα production by human peripheral blood mononuclear cells when exposed to alginate-beads treated with human peritoneal fluid. This elevated responsiveness was not observed with alginate-PLL capsules. The results show that alginate-based capsule surfaces always undergo physicochemical changes of the surface when exposed to human peritoneal fluid. This adsorption may lead to enhancement of the inflammatory responses against the microcapsules. Our result implicate that biocompatibility measurements should not only been done with freshly prepared capsules but also with capsules that have been exposed to fluid from the implantation site in order to predict the in vivo responses. Copyright © 2011 Wiley Periodicals, Inc.
DOCUMENT
A novel synthetic approach to 2,6-bis(acrylamido)pyridine (BAAPy) has been developed, producing a dual-function monomer able to self-crosslink while providing functional binding sites through its amide and pyridine groups. The monomer's structure and purity were confirmed through NMR, FTIR, and HPLC analyses. Poly(BAAPy) was subsequently synthesized and employed in the purification of stilbenes, including t-resveratrol, t-ε-viniferin, and t-piceatannol, all extracted from grape canes. These stilbenes, widely recognized for their antioxidant, antifungal, and antimicrobial properties, are valuable phytochemicals. Grape canes, a by-product of grapevine pruning, serve as a natural and cost-effective source of these bioactive compounds. Using a single-step adsorption–desorption process with an ethanol–water solvent system, the method achieved a 13-fold enrichment of stilbenes with gravimetric purities over 37%. The dual functionality of BAAPy eliminates the need for external crosslinkers and enhances adsorption capacity.
DOCUMENT
De kop van dit artikel is een van de conclusies van Frank Crasborn en Paul Hennissen, die beiden als lerarenopleider en onderwijsadviseur zijn verbonden aan de Fontys Lerarenopleidingen te Sittard. Zij promoveerden op een proefschrift over begeleidingsgedrag van begeleiders van aanstaande leraren.
MULTIFILE
This conference paper deals with various organizations and pilot initiatives regarding the theme of sustainability.
DOCUMENT

Implementation of reliable methodologies allowing Reduction, Refinement, and Replacement (3Rs) of animal testing is a process that takes several decades and is still not complete. Reliable methods are essential for regulatory hazard assessment of chemicals where differences in test protocol can influence the test outcomes and thus affect the confidence in the predictive value of the organisms used as an alternative for mammals. Although test guidelines are common for mammalian studies, they are scarce for non-vertebrate organisms that would allow for the 3Rs of animal testing. Here, we present a set of 30 reporting criteria as the basis for such a guideline for Developmental and Reproductive Toxicology (DART) testing in the nematode Caenorhabditis elegans. Small organisms like C. elegans are upcoming in new approach methodologies for hazard assessment; thus, reliable and robust test protocols are urgently needed. A literature assessment of the fulfilment of the reporting criteria demonstrates that although studies describe methodological details, essential information such as compound purity and lot/batch number or type of container is often not reported. The formulated set of reporting criteria for C. elegans testing can be used by (i) researchers to describe essential experimental details (ii) data scientists that aggregate information to assess data quality and include data in aggregated databases (iii) regulators to assess study data for inclusion in regulatory hazard assessment of chemicals.
DOCUMENT

Bij zijn inauguratie presenteerde Luewton Agostinho een globale visie op watertechnologie, de fysische principes die hierbij betrokken zijn en de uitdagingen, behoeften en conflicten bij het wetenschappelijk en toegepast onderzoek
MULTIFILE
