Methane storage in adsorbed form is a promising way to effectively and safely store fuel for vehicular transportation or for any other potential application. In a solid adsorbent, nanometer wide pores can trap methane by van der Waals forces as high density fluid at low pressure and room temperature. This provides the suitable technology to replace bulky and expensive cylindrical compressed natural gas tanks. Activated carbons with large surface area and high porosity are particularly suitable for methane storage applications at moderate pressures. We study and test the main thermodynamic and kinetic characteristics of methane adsorption and desorption on activated carbon.
DOCUMENT
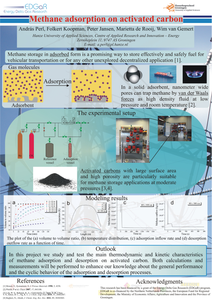
This report presents the experimental and numerical work carried out by ECN and Hanze University of Applied Sciences on methane sorption on activated carbon, as part of their activities within the EDGaR Energy Storage project. Eleven different activated carbon types were tested. It was found that MaxSorb MSC-30 offered the highest methane mass storage density (m/m ratio). However, due to the low density of the MaxSorb MSC-30 activated carbon, the highest volumetric methane storage density (V/V ratio) was found for Brightblack. An increase of the packing density and heat conductivity significantly improves the V/V ratio and shortens the time needed to reach thermal equilibrium. In the case of the Brightblack activated carbon, a total V/V ratio of 112 was found at 12 oC and 40 bar, implying an effective storage density that is 3 times higher than for compressed methane. During the adsorption of methane on activated carbon, sorption heat is released and the temperature of the bed is increased, which negatively affects the effective V/V ratio. Temperature rises up to 70 oC were experimentally observed at higher methane inflow rates. For MaxSorb MSC-30 a temperature rise of 25 oC reduced the effective V/V ratio by about 20 %. The temperature rise of the Brightblack bed caused relatively smaller reductions in the volumetric storage density. Calculations with the validated numerical models indicated an even higher temperature increase for the full scale methane storage, reaching bed temperatures up to 137-150 oC in the case of the MaxSorb MSC-30 activated carbon. At this temperature range, the models indicate a V/V ratio fall down to 46. This performance is similar to the one offered by direct methane compression to 40 bar, and is much lower than the V/V ratio of ~ 100 that was found both experimentally and by calculations for the lab scale reactor performance. The calculations showed, that the low bed permeability can limit the gas flow during adsorption and desorption. A high reactor diameter can countervail the effect of permeability, but the higher dimensions impede the heat dissipation and thus decrease the storage efficiency. Efficient temperature control and management are very important to effectively make use of the methane storage capacity through adsorption.
DOCUMENT
Locally produced methane, - either as biomethane or power-to-gas product, has to be stored to provide a reliable gas source for the fluctuating demand of any local gas distribution network. Additionally, methane is a prominent transportation fuel but its suitability for vehicular application depends on the ability to store an adequate amount in the onboard fuel tank. Adsorption in porous materials could enable a simple, safe and cost-effective method for storing methane at ambient temperature and at reasonably low pressure. In this project we study and test the main thermodynamic and kinetic characteristics of methane adsorption and desorption on activated carbon. Both calculations and measurements are performed to enhance our knowledge about the general performance and the cyclic behavior of the adsorption and desorption processes.
DOCUMENT

Adsorbed natural gas (ANG) storage using metal-organic frameworks (MOFs) is a promising alter- native for efficient natural gas storage at moderate pressures. However, the presence of higher alkanes in natural gas mixtures can significantly affect storage performance by reducing methane adsorption capacity. Basolite C300, a well-studied MOF, offers high volumetric methane storage, but its long-term efficiency in real-world conditions remains a challenge due to potential pore blockage from hydrocarbon accumulation. This study investigates the long-term impact of Cn≥2 alkanes on the adsorption capacity of Basolite C300. Volumetric storage capacities of methane, individual alkanes, and a natural gas mixture were measured at 20 °C. The material underwent 100 adsorption-desorption cycles to assess the progressive impact of Cn≥2 alkanes on methane storage. The experimental results revealed a 63% reduction in methane storage capacity after 100 cycles, highlighting the detrimental effect of alkane accumulation. Higher alkanes were preferentially adsorbed within Basolite C300 micropores, leading to progressive pore blockage and decreased methane uptake. These findings underscore the critical role of gas composition in ANG systems and emphasize the need for mitigation strategies, such as selective pre-adsorption or regeneration techniques, to maintain long-term storage efficiency in MOF-based gas storage applications.
DOCUMENT
Arsenic contamination of groundwater is a major public health concern worldwide. The problem has been reported mainly in southern Asia and, especially, in Bangladesh. Slow-sand filters (SSF) augmented with iron were proven to be a simple, low-cost and decentralized technique for the treatment of arsenic-contaminated sources. In this research, three pilot-scale SSF (flowrate 6 L·h−1) were tested regarding their capability of removing arsenic from groundwater in conditions similar to those found in countries like Bangladesh (70 µg As(III) L−1, 26 °C). From the three, two filters were prepared with mixed media, i.e., sand mixed with corrosive iron matter (CIM filter) and iron-coated sand (ICS filter), and a third conventional SSF was used as a reference. The results obtained showed that the CIM filter could remove arsenic below the World Health Organization (WHO) guideline concentration of 10 µg·L−1, even for inlet concentrations above 150 µg·L−1. After 230 days of continuous operation the arsenic concentration in the effluent started increasing, indicating depletion or saturation of the CIM layer. The effluent arsenic concentration, however, never exceeded the Bangladeshi standard of 50 µg·L−1 throughout the whole duration of the experiments.
DOCUMENT
