Wind and solar power generation will continue to grow in the energy supply of the future, but its inherent variability (intermittency) requires appropriate energy systems for storing and using power. Storage of possibly temporary excess of power as methane from hydrogen gas and carbon dioxide is a promising option. With electrolysis hydrogen gas can be generated from (renewable) power. The combination of such hydrogen with carbon dioxide results in the energy carrier methane that can be handled well and may may serve as carbon feedstock of the future. Biogas from biomass delivers both methane and carbon dioxide. Anaerobic microorganisms can make additional methane from hydrogen and carbon dioxide in a biomethanation process that compares favourably with its chemical counterpart. Biomethanation for renewable power storage and use makes appropriate use of the existing infrastructure and knowledge base for natural gas. Addition of hydrogen to a dedicated biogas reactor after fermentation optimizes the biomethanation conditions and gives maximum flexibility. The low water solubility of hydrogen gas limits the methane production rate. The use of hollow fibers, nano-bubbles or better-tailored methane-forming microorganisms may overcome this bottleneck. Analyses of patent applications on biomethanation suggest a lot of freedom to operate. Assessment of biomethanation for economic feasibility and environmental value is extremely challenging and will require future data and experiences. Currently biomethanation is not yet economically feasible, but this may be different in the energy systems of the near future.
DOCUMENT

With increased share of energy generated from variable renewable sources, storagebecomes a critical issue to ensure constantly balanced supply/demand.Methane is a promising vector for energy storage and transport.
DOCUMENT

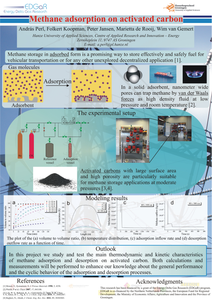
Locally produced methane, - either as biomethane or power-to-gas product, has to be stored to provide a reliable gas source for the fluctuating demand of any local gas distribution network. Additionally, methane is a prominent transportation fuel but its suitability for vehicular application depends on the ability to store an adequate amount in the onboard fuel tank. Adsorption in porous materials could enable a simple, safe and cost-effective method for storing methane at ambient temperature and at reasonably low pressure. In this project we study and test the main thermodynamic and kinetic characteristics of methane adsorption and desorption on activated carbon. Both calculations and measurements are performed to enhance our knowledge about the general performance and the cyclic behavior of the adsorption and desorption processes.
DOCUMENT

This report presents the experimental and numerical work carried out by ECN and Hanze University of Applied Sciences on methane sorption on activated carbon, as part of their activities within the EDGaR Energy Storage project. Eleven different activated carbon types were tested. It was found that MaxSorb MSC-30 offered the highest methane mass storage density (m/m ratio). However, due to the low density of the MaxSorb MSC-30 activated carbon, the highest volumetric methane storage density (V/V ratio) was found for Brightblack. An increase of the packing density and heat conductivity significantly improves the V/V ratio and shortens the time needed to reach thermal equilibrium. In the case of the Brightblack activated carbon, a total V/V ratio of 112 was found at 12 oC and 40 bar, implying an effective storage density that is 3 times higher than for compressed methane. During the adsorption of methane on activated carbon, sorption heat is released and the temperature of the bed is increased, which negatively affects the effective V/V ratio. Temperature rises up to 70 oC were experimentally observed at higher methane inflow rates. For MaxSorb MSC-30 a temperature rise of 25 oC reduced the effective V/V ratio by about 20 %. The temperature rise of the Brightblack bed caused relatively smaller reductions in the volumetric storage density. Calculations with the validated numerical models indicated an even higher temperature increase for the full scale methane storage, reaching bed temperatures up to 137-150 oC in the case of the MaxSorb MSC-30 activated carbon. At this temperature range, the models indicate a V/V ratio fall down to 46. This performance is similar to the one offered by direct methane compression to 40 bar, and is much lower than the V/V ratio of ~ 100 that was found both experimentally and by calculations for the lab scale reactor performance. The calculations showed, that the low bed permeability can limit the gas flow during adsorption and desorption. A high reactor diameter can countervail the effect of permeability, but the higher dimensions impede the heat dissipation and thus decrease the storage efficiency. Efficient temperature control and management are very important to effectively make use of the methane storage capacity through adsorption.
DOCUMENT
Power to methane provides a solution to a couple of two problems: unbalanced production and demand of wind plus solar power electricity and the low methane content of biogas by storing electricity via hydrogen into methane gas using carbon dioxide from biogas and methanogenic bacteria. The four-year project is performed by a consortium of three research institutes and five companies. In WP1 the-state-of- the-art of scientific knowledge on P2M technology is reviewed and evaluated.
DOCUMENT

Methane storage in adsorbed form is a promising way to effectively and safely store fuel for vehicular transportation or for any other potential application. In a solid adsorbent, nanometer wide pores can trap methane by van der Waals forces as high density fluid at low pressure and room temperature. This provides the suitable technology to replace bulky and expensive cylindrical compressed natural gas tanks. Activated carbons with large surface area and high porosity are particularly suitable for methane storage applications at moderate pressures. We study and test the main thermodynamic and kinetic characteristics of methane adsorption and desorption on activated carbon.
DOCUMENT
This Workpackage designs and implements ways of communication on the progress and results of the Power to Methane project to the outside world by means of target group differentiation, communication plan, design of an appropriate project logo and overall incentive to tell the world what we are doing, how and why.
DOCUMENT

Adsorbed natural gas (ANG) storage using metal-organic frameworks (MOFs) is a promising alter- native for efficient natural gas storage at moderate pressures. However, the presence of higher alkanes in natural gas mixtures can significantly affect storage performance by reducing methane adsorption capacity. Basolite C300, a well-studied MOF, offers high volumetric methane storage, but its long-term efficiency in real-world conditions remains a challenge due to potential pore blockage from hydrocarbon accumulation. This study investigates the long-term impact of Cn≥2 alkanes on the adsorption capacity of Basolite C300. Volumetric storage capacities of methane, individual alkanes, and a natural gas mixture were measured at 20 °C. The material underwent 100 adsorption-desorption cycles to assess the progressive impact of Cn≥2 alkanes on methane storage. The experimental results revealed a 63% reduction in methane storage capacity after 100 cycles, highlighting the detrimental effect of alkane accumulation. Higher alkanes were preferentially adsorbed within Basolite C300 micropores, leading to progressive pore blockage and decreased methane uptake. These findings underscore the critical role of gas composition in ANG systems and emphasize the need for mitigation strategies, such as selective pre-adsorption or regeneration techniques, to maintain long-term storage efficiency in MOF-based gas storage applications.
DOCUMENT
Managing dairy excreta as slurry can result in significant emissions of ammonia (NH3) and greenhouse gases (GHGs) during storage and thereafter. Additionally, slurry often has an imbalanced nitrogen (N) to phosphorus (P) ratio for crop fertilization. While various treatments exist to address emissions and nutrient imbalances, each has trade-offs that can result in pollution swapping. An integrated management system, starting with source segregation (SS) in-house to separate faeces and urine into two manageable streams followed by step-wise complementary treatments has been designed to manage nutrients and reduce emissions in the whole chain, but its effect on emissions in storage remains untested. This study investigated NH3, nitrous oxide (N2O), and methane (CH4) emissions and total N losses from integrated storage systems combining SS, mesophilic or thermophilic anaerobic digestion (AD), acidification, drying and zeolite addition and an impermeable cover. These systems were compared to two reference slurry storage systems: in-house uncovered (US) and outside covered (CS). A 30-day lab-scale experiment was conducted at 10 °C, monitoring emissions using an INNOVA1412 gas analyser, while total N losses were assessed using mass balance. Results indicated that the SS fractions treated before covered storage exhibited significantly lower emissions (NH3 or CH4 or both) compared to both reference slurry storage systems (US and CS). Source segregation combined with acidification of urine and AD of faeces at 35 °C and an impermeable cover allowed for a 99% reduction in NH3 emissions, a 45% reduction in CH4 emissions and had no effect on N2O emissions as compared to US. When AD of faeces was conducted at 55 °C instead of 35 °C, the CH4 emission was reduced by 77% compared to US. This study concludes that SS combined with urine and faeces treatment allows a more effective and simultaneous reduction of all emissions in storage as compared to slurry storage systems, while also effectively separating nutrients allowing more precise N and P fertilization with dairy excreta. Further research is necessary to assess emissions and fertilizer value of treated fractions after field application, in addition to the associated costs.
LINK
The project BioP2M came to a close in June 2019 after a consortium of stakeholders in the field of energy transition worked together to research the diverse role of Methane. In this report the results are presented and future plans are discussed.
DOCUMENT
